May 11, 2022From the table of available reagents, select the one (s) you would use to accomplish the transformations shown below: Reagents Available: a. CrO3, H2SO4, H2O b. H2, Pd c. NaBH4 d. O3, then Zn, CH3CO2H e. KMnO4, H2O f. LiAlH4, then H3O+ g. Dess-Martin Periodinane Use the minimum number of steps; in no case are more than two steps necessary.
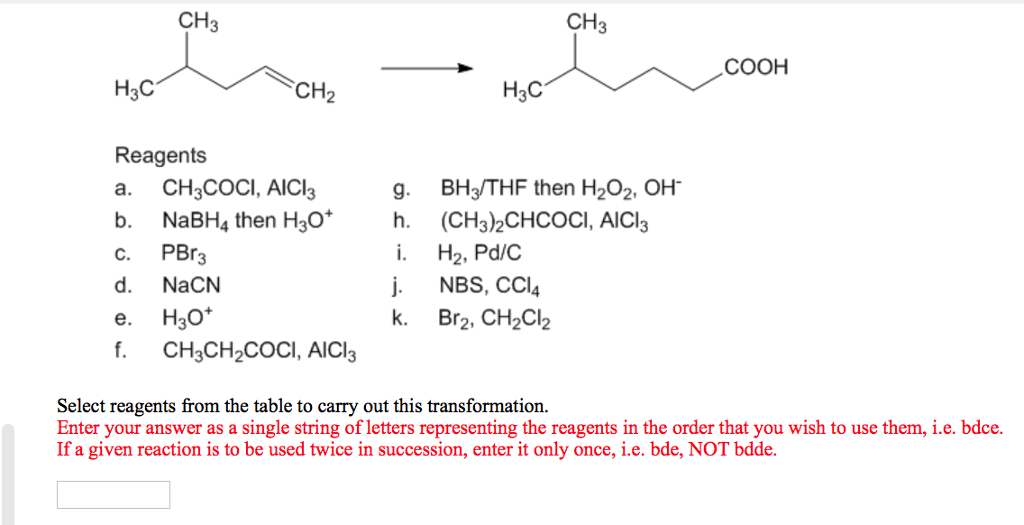
Solved CH3 CH3 H3C CH2 Reagents a. CH3COCI, AICI3 b. NaBH4 | Chegg.com
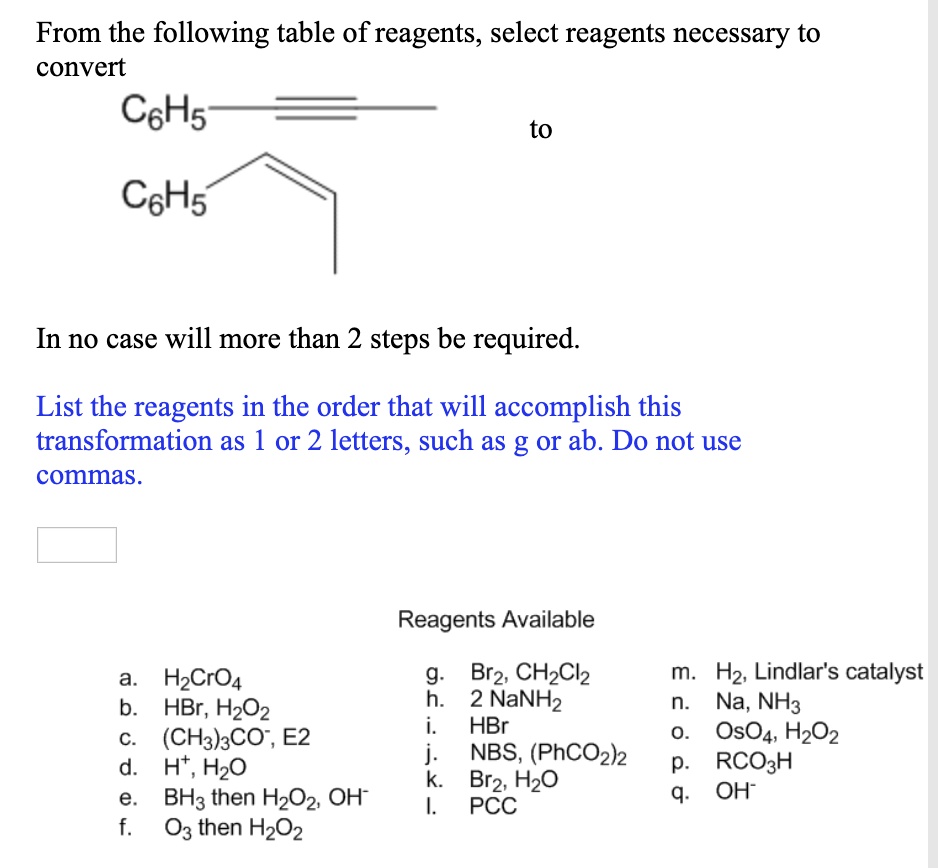
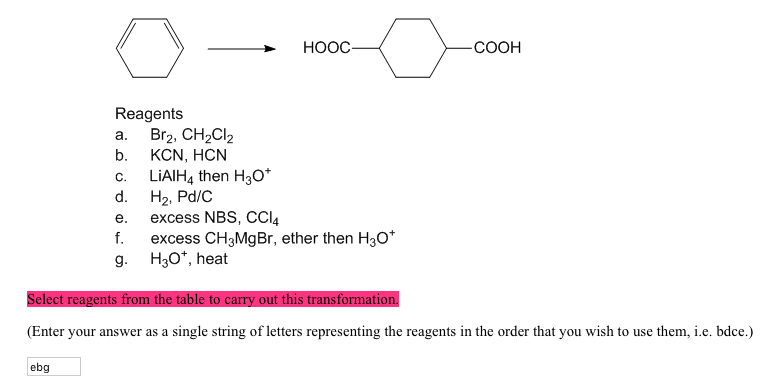
Br2, CH2Cl2 b. KCN, HON C. LiAlHA then H3Of d. H2, Pd/C e. excess NBS, CCI4 f. excess CH3MgBr, ether then HaO* g. H30*, heat Select reagents from the table to carry out this transformation. (Enter your answer as a single string of letters representing the reagents in the order that you wish to use them, i.e. bdce.)
Source Image: numerade.com
Download Image
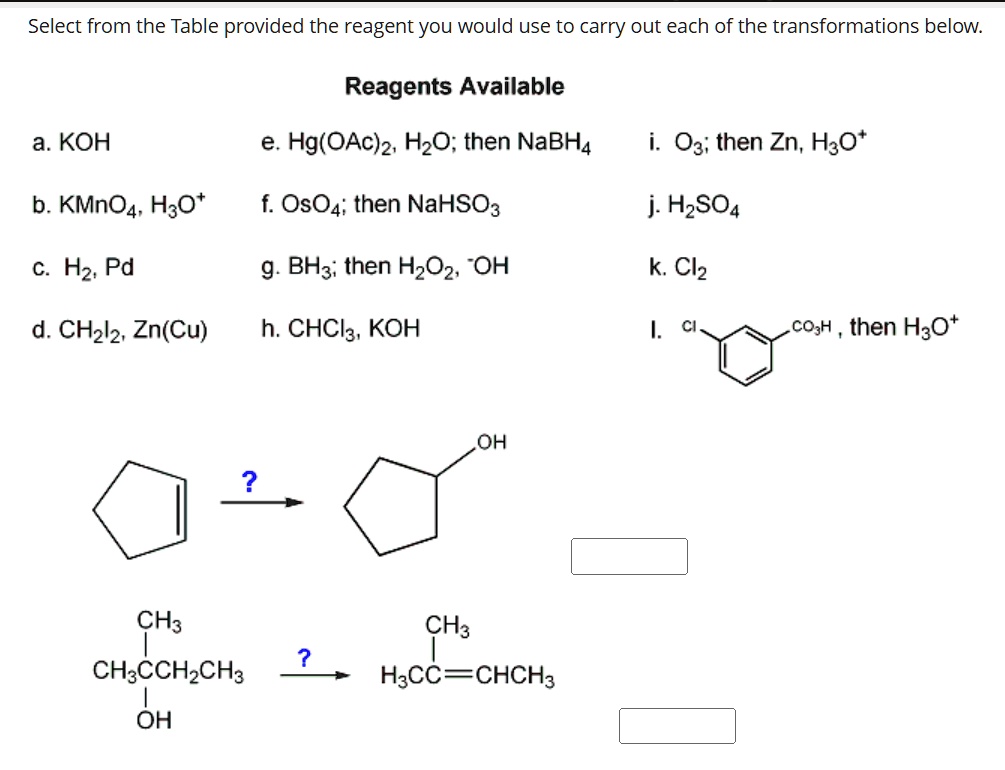
Select from the Table provided the reagent you would use to carry out each of the transformations below. Reagents Available а. КОН e. Hg (OAc)2, H2O; then NaBH4 i. O3; then Zn, H3O* b. KMNO4, H3O* f. Os04; then NaHSO3 j. H2SO4 С. На, Рd g. BH3; then H2O2, “OH k. Cl2 d. CH212, Zn (Cu) h.

Source Image: shutterstock.com
Download Image
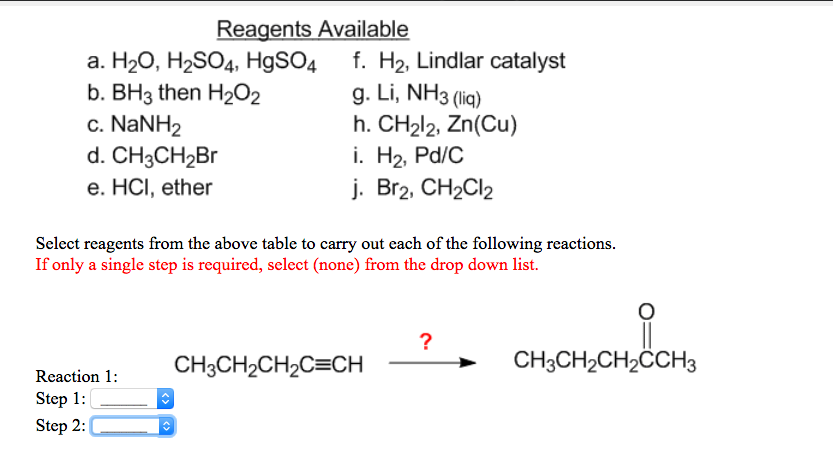
Spring 2018 SURF posters by kccstem – Issuu NBS, CCl4 h. CrO3, H2SO4 Select reagents from the table to show how you would carry out this transformation. Ifmore than one route exists, use the one that requires the fewest steps; in no case will more than 3 steps be required Enter your selection as a series of letters for the reagents in the order that you wish to use them, ie. fca.
Source Image: numerade.com
Download Image
Select Reagents From The Table To Carry Out This Transformation
NBS, CCl4 h. CrO3, H2SO4 Select reagents from the table to show how you would carry out this transformation. Ifmore than one route exists, use the one that requires the fewest steps; in no case will more than 3 steps be required Enter your selection as a series of letters for the reagents in the order that you wish to use them, ie. fca. Jul 11, 2023Specify the reagents you would use to carry out the conversion by using letters from the table. The reaction may require more than one reagent. If so, write the letters in the order that they are used, e.g.r ef. If two or more ways of conversion to the same. product are possible, show only one of them. The reagents are: 1 item atternpt remaining
SOLVED: From the following table of reagents, select reagents necessary to convert C6H6 to C6H5CH3. In no case will more than 2 steps be required. List the reagents in the order that
From the Table of Reagents, select those that would be used to carry out the following transformation: Synthesis of Organic Compounds: The hydrogens of the benzene ring are replaced by Science & Lab Tables – Tables – Furniture & Spaces

Source Image: nascoeducation.com
Download Image
Solved Select reagents from the above table to carry out | Chegg.com From the Table of Reagents, select those that would be used to carry out the following transformation: Synthesis of Organic Compounds: The hydrogens of the benzene ring are replaced by
Source Image: chegg.com
Download Image
Solved CH3 CH3 H3C CH2 Reagents a. CH3COCI, AICI3 b. NaBH4 | Chegg.com May 11, 2022From the table of available reagents, select the one (s) you would use to accomplish the transformations shown below: Reagents Available: a. CrO3, H2SO4, H2O b. H2, Pd c. NaBH4 d. O3, then Zn, CH3CO2H e. KMnO4, H2O f. LiAlH4, then H3O+ g. Dess-Martin Periodinane Use the minimum number of steps; in no case are more than two steps necessary.
Source Image: chegg.com
Download Image
Spring 2018 SURF posters by kccstem – Issuu Select from the Table provided the reagent you would use to carry out each of the transformations below. Reagents Available а. КОН e. Hg (OAc)2, H2O; then NaBH4 i. O3; then Zn, H3O* b. KMNO4, H3O* f. Os04; then NaHSO3 j. H2SO4 С. На, Рd g. BH3; then H2O2, “OH k. Cl2 d. CH212, Zn (Cu) h.

Source Image: issuu.com
Download Image
Organic ALL RXN Table 2 | PDF | Alcohol | Aldehyde Select from the Table provided the reagent you would use to carry out each of the transformations below. Reagents Available а. КОН e. Hg (OAc)2, H2O; then NABH4 i. O3; then Zn, H3o* b. KMNO4, H3O* f. OsO4; then NaHSO3 j. H2SO4 с. На, Рd g. BH3; then H2O2, “OH k. Cl2 d. CH2l2, Zn (Cu) h. CНCl3, КОН I. C. cO,H , then H3O* но OH H3C- H3C- H3C H3C

Source Image: scribd.com
Download Image
Solved Select reagents from the table to carry out this | Chegg.com NBS, CCl4 h. CrO3, H2SO4 Select reagents from the table to show how you would carry out this transformation. Ifmore than one route exists, use the one that requires the fewest steps; in no case will more than 3 steps be required Enter your selection as a series of letters for the reagents in the order that you wish to use them, ie. fca.
Source Image: chegg.com
Download Image
Solved Select reagents from the table to carry out this | Chegg.com Jul 11, 2023Specify the reagents you would use to carry out the conversion by using letters from the table. The reaction may require more than one reagent. If so, write the letters in the order that they are used, e.g.r ef. If two or more ways of conversion to the same. product are possible, show only one of them. The reagents are: 1 item atternpt remaining
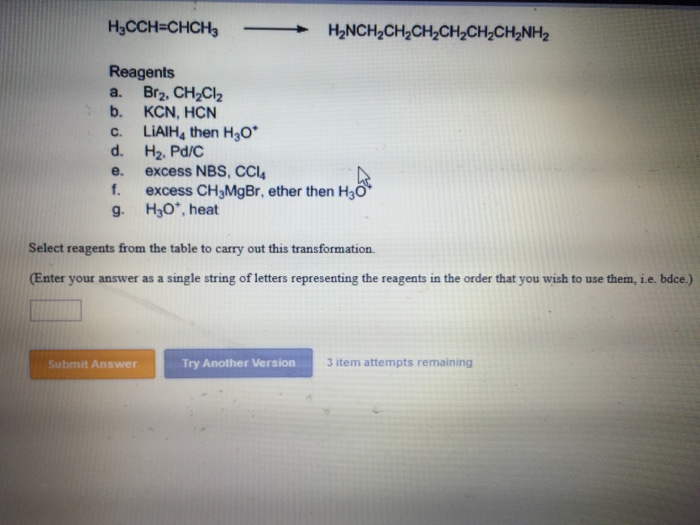
Source Image: chegg.com
Download Image
Solved Select reagents from the above table to carry out | Chegg.com
Solved Select reagents from the table to carry out this | Chegg.com Br2, CH2Cl2 b. KCN, HON C. LiAlHA then H3Of d. H2, Pd/C e. excess NBS, CCI4 f. excess CH3MgBr, ether then HaO* g. H30*, heat Select reagents from the table to carry out this transformation. (Enter your answer as a single string of letters representing the reagents in the order that you wish to use them, i.e. bdce.)
Spring 2018 SURF posters by kccstem – Issuu Solved Select reagents from the table to carry out this | Chegg.com Select from the Table provided the reagent you would use to carry out each of the transformations below. Reagents Available а. КОН e. Hg (OAc)2, H2O; then NABH4 i. O3; then Zn, H3o* b. KMNO4, H3O* f. OsO4; then NaHSO3 j. H2SO4 с. На, Рd g. BH3; then H2O2, “OH k. Cl2 d. CH2l2, Zn (Cu) h. CНCl3, КОН I. C. cO,H , then H3O* но OH H3C- H3C- H3C H3C