Exercise 2.6.1 2.6. 1. Draw the resonance contributors that correspond to the curved, two-electron movement arrows in the resonance expressions below. Then identify the type of resonance motion in each structure below. Answer. Exercise 2.6.2 2.6. 2. In each resonance expression, identify the type of resonance motion.
PPT – Molecular Geometry and Bonding Theories PowerPoint Presentation, free download – ID:9093593
Step 1: Draw the Lewis dot structure of NO 3-, as shown below. The procedure is discussed in our Lewis Structure article. The image shows that NO 3- has two oxygen atoms (O1 and O3) with three lone pairs. Also, these two oxygen atoms have a -1 charge and are single bonded to the nitrogen atom.
Source Image: chegg.com
Download Image
Minor resonance structures are all the resonance contributors that are higher in energy than the lowest-energy contributor. For example, we can draw three possible contributors for formamide, HCONH₂. We have to decide which of these is the lowest-energy form. That one will be the major contributor. All the others will be minor contributors.

Source Image: sciencedirect.com
Download Image
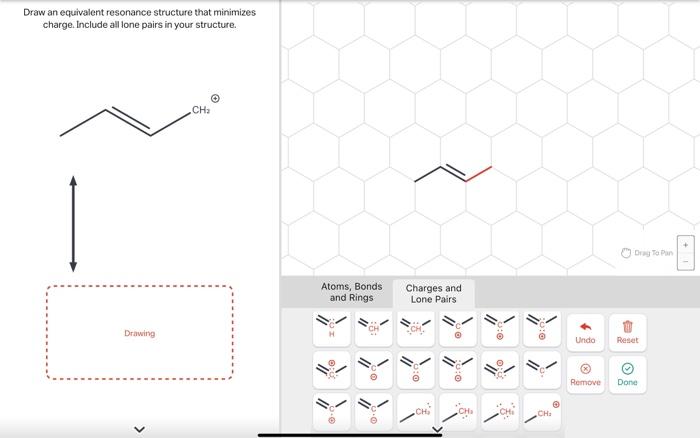
Solved Draw an equivalent resonance structure that minimizes | Chegg.com Evaluating The Resonance Forms Of Ethene (“Ethylene”) The simplest molecule with a π bond is ethene. If we draw a resonance structure for it, we can move the π bond to the lone pair of one of the end carbons (doesn’t matter which one) to give a carbocation and a lone pair. This is a “legal” resonance form, since we’re not breaking

Source Image: m.youtube.com
Download Image
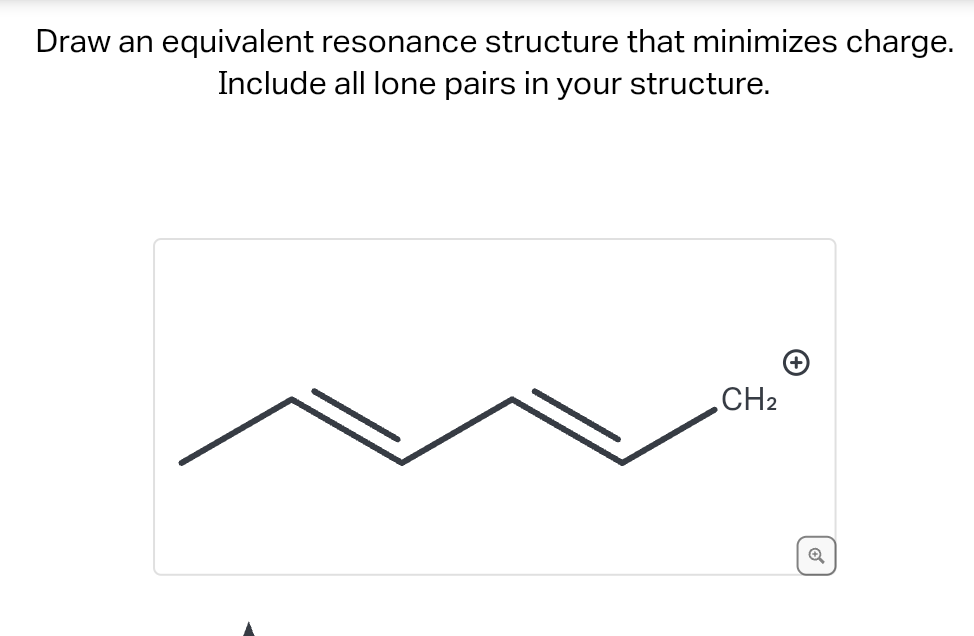
Draw An Equivalent Resonance Structure That Minimizes Charge
Evaluating The Resonance Forms Of Ethene (“Ethylene”) The simplest molecule with a π bond is ethene. If we draw a resonance structure for it, we can move the π bond to the lone pair of one of the end carbons (doesn’t matter which one) to give a carbocation and a lone pair. This is a “legal” resonance form, since we’re not breaking Question Ff.274. Transcribed Image Text: Draw an equivalent resonance structure that minimizes charge. Include all lone pairs in your structure. Drawing CH2 Expert Solution Trending now This is a popular solution! Step by step Solved in 2 steps with 1 images SEE SOLUTION Check out a sample Q&A here Knowledge Booster Learn more about
Picking the best non-equivalent resonance structure using formal charge – YouTube
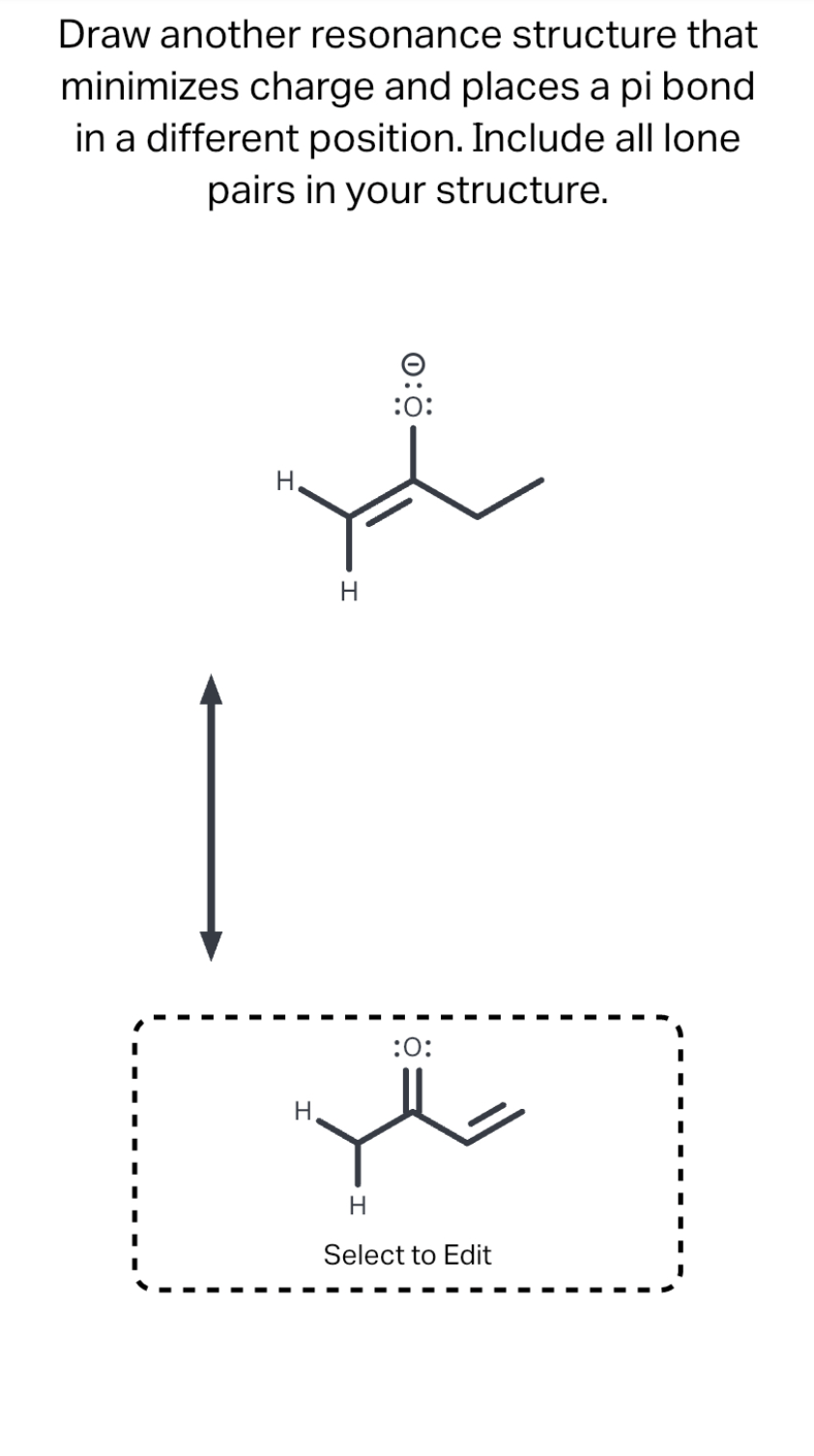
1) There is ONLY ONE REAL STRUCTURE for each molecule or ion. This real structure (the resonance hybrid) takes its character from the average of all the individual resonance contributors. When looking at a resonance contributors, we are seeing the exact same molecule or ion depicted in different ways. Solved Draw another resonance structure that minimizes | Chegg.com
Source Image: chegg.com
Download Image
Solved Draw an equivalent resonance structure that minimizes | Chegg.com 1) There is ONLY ONE REAL STRUCTURE for each molecule or ion. This real structure (the resonance hybrid) takes its character from the average of all the individual resonance contributors. When looking at a resonance contributors, we are seeing the exact same molecule or ion depicted in different ways.
Source Image: chegg.com
Download Image
PPT – Molecular Geometry and Bonding Theories PowerPoint Presentation, free download – ID:9093593 Exercise 2.6.1 2.6. 1. Draw the resonance contributors that correspond to the curved, two-electron movement arrows in the resonance expressions below. Then identify the type of resonance motion in each structure below. Answer. Exercise 2.6.2 2.6. 2. In each resonance expression, identify the type of resonance motion.
Source Image: slideserve.com
Download Image
Solved Draw an equivalent resonance structure that minimizes | Chegg.com Minor resonance structures are all the resonance contributors that are higher in energy than the lowest-energy contributor. For example, we can draw three possible contributors for formamide, HCONH₂. We have to decide which of these is the lowest-energy form. That one will be the major contributor. All the others will be minor contributors.
Source Image: chegg.com
Download Image
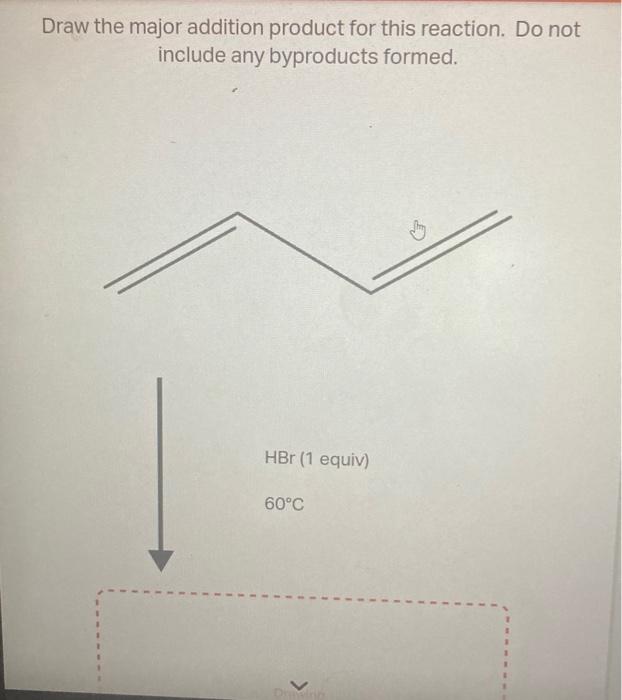
ILLUSTRATION ART: ONE LOVELY DRAWING, part 57 This video desribes how to draw and distinguish between equivalent and non-equivalent resonance structures. Formal charge calculation is also discussed.
Source Image: illustrationart.blogspot.com
Download Image
Isotachophoresis: Theory and Microfluidic Applications | Chemical Reviews Evaluating The Resonance Forms Of Ethene (“Ethylene”) The simplest molecule with a π bond is ethene. If we draw a resonance structure for it, we can move the π bond to the lone pair of one of the end carbons (doesn’t matter which one) to give a carbocation and a lone pair. This is a “legal” resonance form, since we’re not breaking

Source Image: pubs.acs.org
Download Image
Isotachophoresis: Theory and Microfluidic Applications | Chemical Reviews Question Ff.274. Transcribed Image Text: Draw an equivalent resonance structure that minimizes charge. Include all lone pairs in your structure. Drawing CH2 Expert Solution Trending now This is a popular solution! Step by step Solved in 2 steps with 1 images SEE SOLUTION Check out a sample Q&A here Knowledge Booster Learn more about

Source Image: pubs.acs.org
Download Image
Solved Draw an equivalent resonance structure that minimizes | Chegg.com
Isotachophoresis: Theory and Microfluidic Applications | Chemical Reviews Step 1: Draw the Lewis dot structure of NO 3-, as shown below. The procedure is discussed in our Lewis Structure article. The image shows that NO 3- has two oxygen atoms (O1 and O3) with three lone pairs. Also, these two oxygen atoms have a -1 charge and are single bonded to the nitrogen atom.
Solved Draw an equivalent resonance structure that minimizes | Chegg.com Isotachophoresis: Theory and Microfluidic Applications | Chemical Reviews This video desribes how to draw and distinguish between equivalent and non-equivalent resonance structures. Formal charge calculation is also discussed.